Research
Find more about our research at the Nouailles Lab!
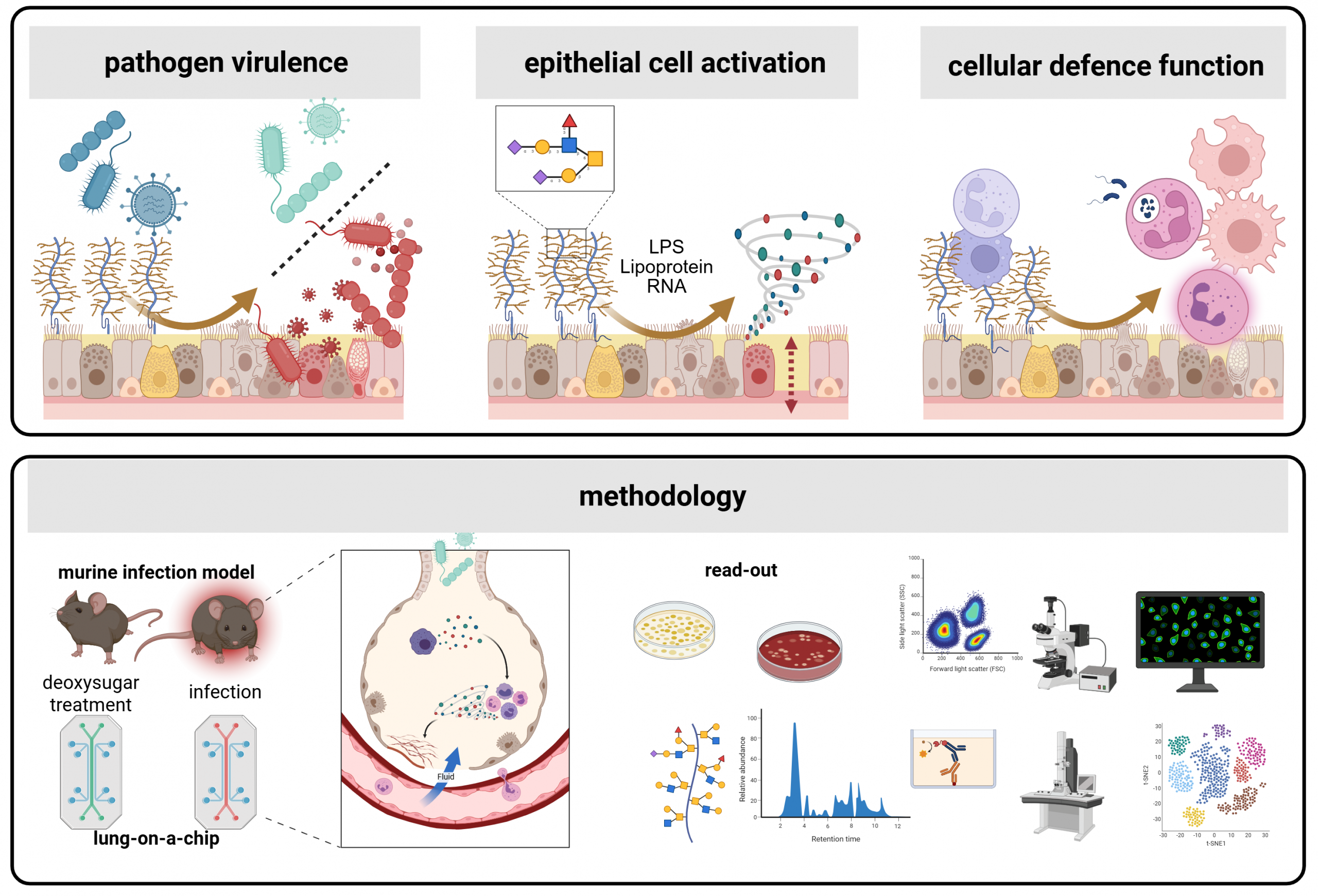
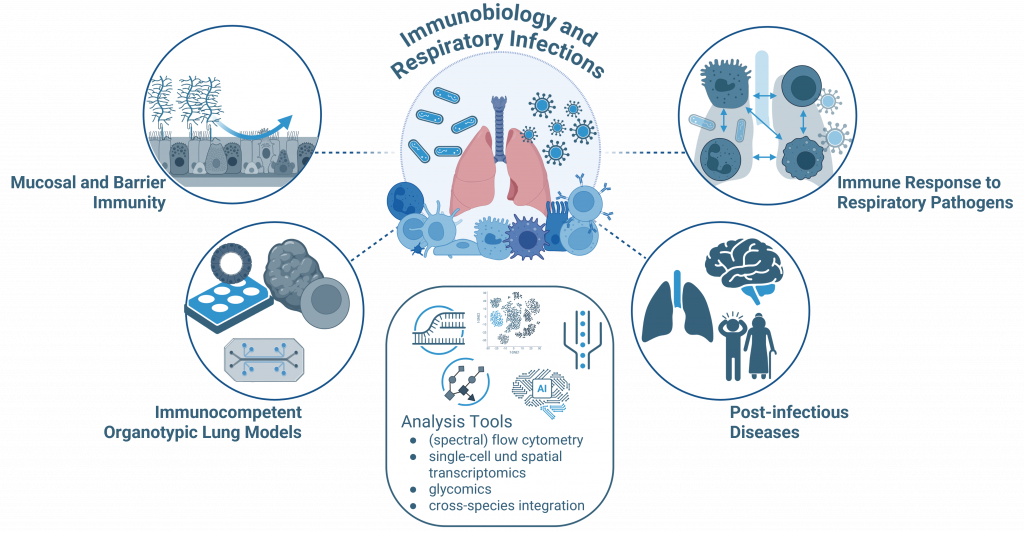
In this research area we investigate mucosal and barrier immunity in the lungs under both healthy and diseased conditions. Our main focus is the epithelial glycocalyx, particularly mucins, as well as the roles of resident and recruited immune cells and their interplay with both the epithelium and the glycocalyx.
To dissect these interactions, we employ molecular, glycoengineering and imaging approaches that enable high-resolution analysis of barrier function, immune activation, and the epithelial glycome. Through these studies, we aim to reveal how the mucosal barrier, its sugars and cells, regulate immunity and inflammation, providing insights into mechanisms of protection and pathology in the respiratory tract.
Project and related links
CRC 1449 Dynamic Hydrogels at Biointerfaces
Roland und Elfriede Schauer Stiftung
Project members
Geraldine Nouailles
Karen Hoffmann
Niall Hardiman
Raquel Alonso Roman
Sebastian Schickinger
Marlen Lapson
Ulrike Behrendt
Alina Nettesheim (Alumni)
Kerstin Linke (Alumni)
Cengiz Gökeri (Alumni)
Main cooperation partners
Kevin Pagel and team
Matthias Ochs and team
Elena Lopez-Rodriguez and team
Peter Seeberger and team
Wolfgang Kübler and team
Martin Witzenrath / Cengiz Gökeri
Publications
Hoffmann K, Behrendt U, Pennitz P, Kirsten H, Pohl J, Lopez-Rodriguez E, Weissfuss C, Kollmeier J, Tönnies M, Brill S, Steinestel K, Witzenrath M, Wenzel W, Zobel C’*, Nouailles G*. Mechanical strain exacerbates Pseudomonas infection in an organoid-based pneumonia-on-a-chip model. J Clin Invest. 2025 Nov 18:e192454. * shared last.
Berger S#, Goekeri C#, Pennitz P#, Gutbier B, Michalick L, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Wienhold SM, Behrendt U, Taylor A, Dietert K, Kirsten H, Kunder S, Mueller K, Weigel M, Hain T, Volkers SM, Weis S, Gruber AD, Sander LE, Kuebler WM, CAPSyS Study Group, Witzenrath M, Nouailles G. Neutrophil-chemoattractant CXCL5 increases lung barrier permeability in acute lung injury. Mucosal Immunol. 2025 Oct 10:S1933-0219(25)00105-9. #shared first.
Goekeri C#, Linke KAK#, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger SMK, Zobel CM, Pagel K, Gruber AD, Ochs M, Witzenrath M, Nouailles G. Enzymatic Modulation of the Pulmonary Glycocalyx Enhances Susceptibility to Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2024;71(6):646-658. #shared first.
Berger S*, Goekeri C*, Pennitz P*, Gutbier B, Michalick L, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Wienhold SM, Behrendt U, Taylor A, Dietert K, Kirsten H, Kunder S, Mueller K, Weigel M, Hain T, Volkers SM, Weis S, Gruber AD, Sander LE, Kuebler WM, CAPSyS study group, Witzenrath M, Nouailles G. Neutrophil-chemoattractant CXCL5 induces lung barrier permeability in acute lung injury. bioRxiv. 2024. *shared first
Goekeri C#, Linke KAK#, Hoffmann K, Lopez-Rodriguez E, Gluhovic, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger SMK, Zobel CM, Pagel K, Gruber AD, Ochs M, Witzenrath M, Nouailles G. Enzymatic modulation of the pulmonary glycocalyx alters susceptibility to Streptococcus pneumoniae. bioRxiv. 2024.01.03.573996. #shared first.
Herbst CJ, Lopez-Rodriguez E, Gluhovic V, Schulz S, Brandt R, Timm S, Abledu J, Falivene J, Pennitz P, Kirsten H, Nouailles G, Witzenrath M, Ochs M, Kuebler WM. Characterization of Commercially Available Human Primary Alveolar Epithelial Cells. Am J Respir Cell Mol Biol. 2024;70(5):339-350.
Timm S, Lettau M, Hegermann J, Rocha ML, Weidenfeld S, Fatykhova D, Gutbier B, Nouailles G, Lopez-Rodriguez E, Hocke A, Hippenstiel S, Witzenrath M, Kuebler WM, Ochs M. The unremarkable alveolar epithelial glycocalyx: a thorium dioxide-based electron microscopic comparison after heparinase or pneumolysin treatment. Histochem Cell Biol. 2023;160(2):83-96.
Ochs M, Hegermann J, Lopez-Rodriguez E, Timm S, Nouailles G, Matuszak J, Simmons S, Witzenrath M, Kuebler WM. On Top of the Alveolar Epithelium: Surfactant and the Glycocalyx. Int J Mol Sci. 2020;21(9):3075.
Conference Papers
Jenner Glycobiology and Medicine Symposium 2025, 10. – 13 June 2025, Maynooth, Ireland. Goekeri C, Schickinger S, Nettesheim A, Linke K, Bechtella L, Vos G, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Bath J, Ribbeck K, Pagel K, Gruber A, Ochs M, Seeberger P, Witzenrath M, Nouailles G. Unraveling the Role of Terminal Fucosylation in Pneumococcal Pneumonia
Lung Science Conference (ERS), 20. – 23 March 2025, Estoril, Portugal. Goekeri C, Linke K, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger S, Zobel C, Bechtella L, Vos G, Pagel K, Gruber A, Ochs M, Witzenrath M, Nouailles G. Pulmonary glycosaminoglycans and fucosylation regulate lung barrier function and defense against S. pneumoniae
28th Joint Symposium: Infection and Immune Defense, 24. – 26 March 2025, Burg Rothenfels, Germany. Goekeri C, Schickinger S, Nettesheim A, Linke K, Bechtella L, Vos G, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Bath J, Ribbeck K, Pagel K, Gruber A, Ochs M, Seeberger P, Witzenrath M, Nouailles G. Inhibition of pulmonary fucosylation alleviates pneumococcal pneumonia
28th Joint Symposium: Infection and Immune Defense, 24. – 26 March 2025, Burg Rothenfels, Germany. Hardiman N, Goekeri C, Linke K, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger S, Zobel C, Pagel K, Gruber A, Ochs M, Witzenrath M, Nouailles G. Enzymatic Modulation of the Pulmonary Glycocalyx Enhances Susceptibility to Streptococcus pneumoniae
ERS Congress 2024, 7. – 11 September 2024, Vienna, Austria. Goekeri C, Schickinger SMK, Nettesheim A, Linke KAK, Bechtella L, Vos G, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Bath J, Ribbeck K, Pagel K, Gruber AD, Ochs M, Seeberger PH, Witzenrath M, Nouailles G. Unraveling the role of α-(1,2)-fucosylation in bacterial pneumonia. European Respiratory Journal. 2024 64(suppl 68): PA2395
ATS Conference 2024, 17. – 22 May 2024, San Diego, USA. Goekeri C, Nettesheim A, Schickinger SMK, Linke KAK, Behrendt U, Barthel D, Lopez-Rodriguez E, Gluhovic V, Schmidt K, Bechtella L, Voß A, Kunder S, Pagel K, Gruber AD, Hedtrich S, Ochs M, Seeberger PH, Witzenrath M, Nouailles G. Elucidating the Role of Epithelial α-(1,2)-fucosylation in Pneumococcal Virulence. American Journal of Respiratory and Critical Care Medicine 2024;209:A4360
ERS International Congress 2023, 09.-13.09.2023, Milan. Goekeri C, Nettesheim A, Linke KAK, Schickinger SMK, Behrendt U, Barthel D, Pennitz P, Lopez-Rodriguez E, Gluhovic V, Schmidt K, Bechtella L, Voß A, Kunder S, Pagel K, Gruber AD, Hedtrich S, Ochs M, Witzenrath M, Nouailles G. Deciphering the role of pulmonary α-(1,2)-fucosylation in pneumococcal pneumonia. Eur Respir J. Sep 2023, 62 (suppl 67) PA1098.
1st International Symposium of the CRC 1449 on “Dynamic hydrogels at biointerfaces”, 06.-07.10.2022, Berlin. Linke K, Goekeri C, Nettesheim A, Behrendt U, Lopez-Rodriguez E, Ochs M, Witzenrath M, and Nouailles G. – Epithelial glycocalyx and bacterial pneumonia.
1st International Symposium of the CRC 1449 on “Dynamic hydrogels at biointerfaces”, 06.-07.10.2022, Berlin. Nettesheim A, Linke K, Behrendt U, Barthel D, Lopez-Rodriguez E, Ochs M, Gorenflos López J L, Hackenberger C P R, Seeberger P H, Witzenrath M, Goekeri C, and Nouailles G. – Characterizing the role of fucosylation in bacterial pneumonia.
Editorials and Press
Highlights/Papers by Junior Investigators in December 2024 issue of AJRCMB
Editorial comment for Goekeri et al. 2024
Katharina Ribbeck in Tagesspiegel supplement, issue of 3.10.2022
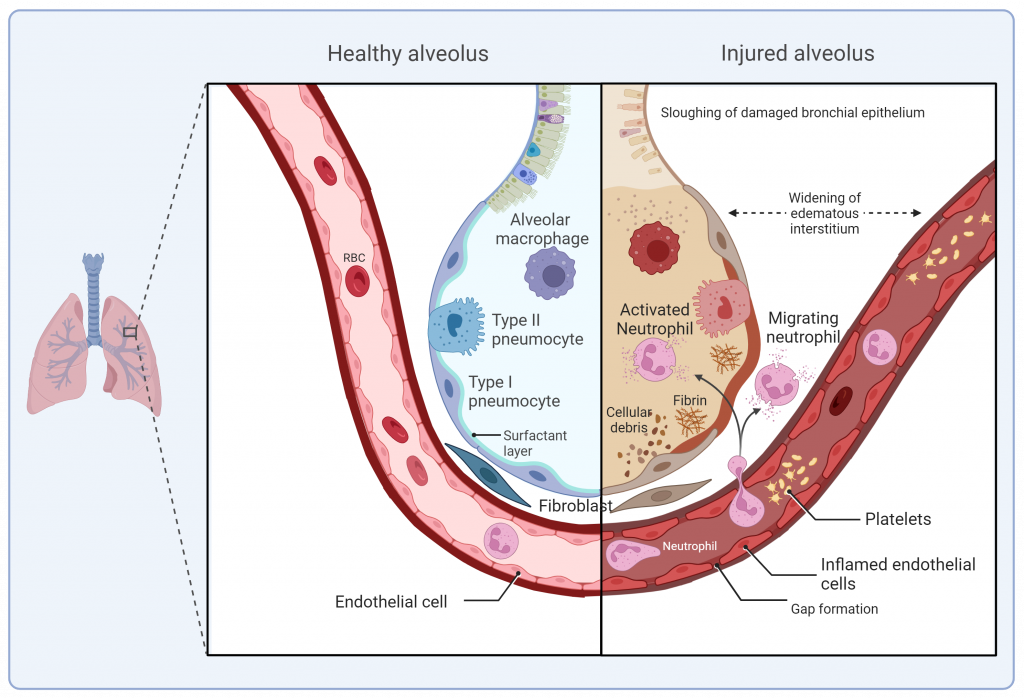
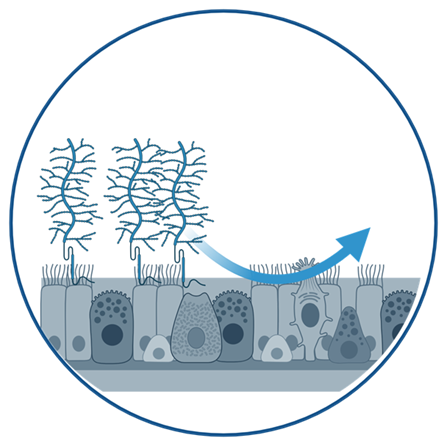
In this research area we develop advanced in vitro lung models based on primary human or patient-derived cells to emulate the human lungs and their response to disease. We design and refine air-liquid interface cultures that maintain a differentiated epithelial layer, as well as alveolar and bronchial organoids that provide a structural and functional 3D environment. In addition, we use lung-on-a-chip platforms that recapitulate the alveolar-capillary barrier. By incorporating both innate and adaptive immune cells, these models provide a physiologically and immune-competent setting.
Our goal is to create translationally relevant platforms to investigate host-pathogens interactions, immune responses, and therapeutic interventions under conditions that closely mimic the human lung environment.
Project and related links
NoVAP Lab Bundeswehr Krankenhaus
Project members
Geraldine Nouailles
Karen Hoffmann
Raquel Roman
Sebastian Schickinger
Ulrike Behrendt
Peter Pennitz
Louis Jage-Bowler
Anne Angela Poletti
Lisa Pfeiffer (Alumni)
Cengiz Gökeri (Alumni)
Main cooperation partners
Christian Zobel, Werner Wenzel and team
Elena Lopez-Rodriguez and team
Leif-Erik Sander, Philipp Georg and team
Project-related publications
Hoffmann K, Behrendt U, Pennitz P, Kirsten H, Pohl J, Lopez-Rodriguez E, Weissfuss C, Kollmeier J, Tönnies M, Brill S, Steinestel K, Witzenrath M, Wenzel W, Zobel C*, Nouailles G*. Mechanical strain exacerbates Pseudomonas infection in an organoid-based pneumonia-on-a-chip model. J Clin Invest. 2025 Nov 18:e192454. *shared last.
Alonso-Roman R, Mosig AS, Figge MT, Papenfort K, Eggeling C, Schacher FH, Hube B, Gresnigt MS. Organ-on-chip models for infectious disease research. Nat Microbiol. 2024 Apr;9(4):891-904. doi: 10.1038/s41564-024-01645-6. Epub 2024 Mar 25.
Goekeri C#, Linke KAK#, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger SMK, Zobel CM, Pagel K, Gruber AD, Ochs M, Witzenrath M, Nouailles G. Enzymatic Modulation of the Pulmonary Glycocalyx Enhances Susceptibility to Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2024;71(6):646-658. # shared first
Hoffmann K, Obermayer B, Hönzke K, Fatykhova D, Demir Z, Löwa A, Alves LGT, Wyler E, Lopez-Rodriguez E, Mieth M, Baumgardt M, Hoppe J, Firsching TC, Tönnies M, Bauer TT, Eggeling S, Tran HL, Schneider P, Neudecker J, Rückert JC, Gruber AD, Ochs M, Landthaler M, Beule D, Suttorp N, Hippenstiel S, Hocke AC, Kessler M. Human alveolar progenitors generate dual lineage bronchioalveolar organoids. Commun Biol. 2022;5(1):875.
Hoffmann K, Berger H, Kulbe H, Thillainadarasan S, Mollenkopf HJ, Zemojtel T, Taube E, Darb-Esfahani S, Mangler M, Sehouli J, Chekerov R, Braicu EI, Meyer TF, Kessler M. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 2020;39(6):e104013.
Conference Papers
EMBL – Infection: pathogens, hosts, and microbiomes, EMBO, 26. – 29 May 2025, Heidelberg, Germany. Hoffmann K, Goekeri C, Behrendt U, Pohl J, Lopez-Rodriguez E, Weissfuss C, Kollmeier J, Tönnies M, Brill S, Steinestel K, Witzenrath M, Wenzel W, Zobel C, Nouailles G. Pneumonia-on-a-chip models highlight the crucial role of the glycocalyx and mechanical strain in regulating susceptibility to bacterial infections.
Human 3D Organ Models Networking Event of Charité 3R, 14 November 2024, Berlin, Germany. Hoffmann K, Weißfuß C, Goekeri C, Zobel C, Witzenrath M, Nouailles G.– In vitro models to study bacterial pneumonia.
DZL Annual Meeting, 05.06. – 07.06.2024, Bad Nauheim. Goekeri C, Linke KAK, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Voß A, Kunder S, Zappe A, Timm S, Nettesheim A, Schickinger SMK, Zobel CM, Pagel K, Gruber AD, Ochs M, Witzenrath M, Geraldine Nouailles. Enzymatic modulation of the pulmonary glycocalyx alters susceptibility to Streptococcus pneumoniae.
Si-Mposium, 12.09.2024, Berlin. Hoffmann K, Goekeri C, Zobel CM, Witzenrath M, Nouailles G. Alveolus-on-a-chip for pneumonia research.
ERS Congress, 07.09. – 11.09.2024, Vienna. Hoffmann K, Goekeri C, Obermayer-Wasserscheid B, Hocke AC, Kessler M, Zobel CM, Witzenrath M and Nouailles G. Advanced in vitro models for pneumonia research.
63rd Congress of the DGP, 29.03 – 01.04.2023, Düsseldorf. Pfeiffer L, Weißfuß C, Behrendt U, Wienhold S, Gutbier B, Krishnamoorthy G, Witzenrath M, Nouailles G, Goekeri C, Pennitz P. Approaching xeno-free cultivation and utilization of pulmonary pathogens in vitro.
International Conference of the SFB-TR 84, 8. – 10.09.2022, Berlin. Pfeiffer L, Weißfuß C, Behrendt U, Wienhold S, Gutbier B, Krishnamoorthy G, Witzenrath M, Nouailles G, Goekeri C, Pennitz P. – Approaching xeno-free cultivation and utilization of pulmonary pathogens in vitro.
2nd Summer School on „Infection Biology“ of the Universitöt Greifswald and FLI, 26. – 28.09.2022, Greifswald. Pfeiffer L, Weißfuß C, Behrendt U, Wienhold S, Gutbier B, Krishnamoorthy G, Witzenrath M, Nouailles G, Goekeri C, Pennitz P. – Approaching xeno-free cultivation of pulmonary pathogens in vitro.
Editorial and press
Highlights/Papers by Junior Investigators in December 2024 issue of AJRCMB
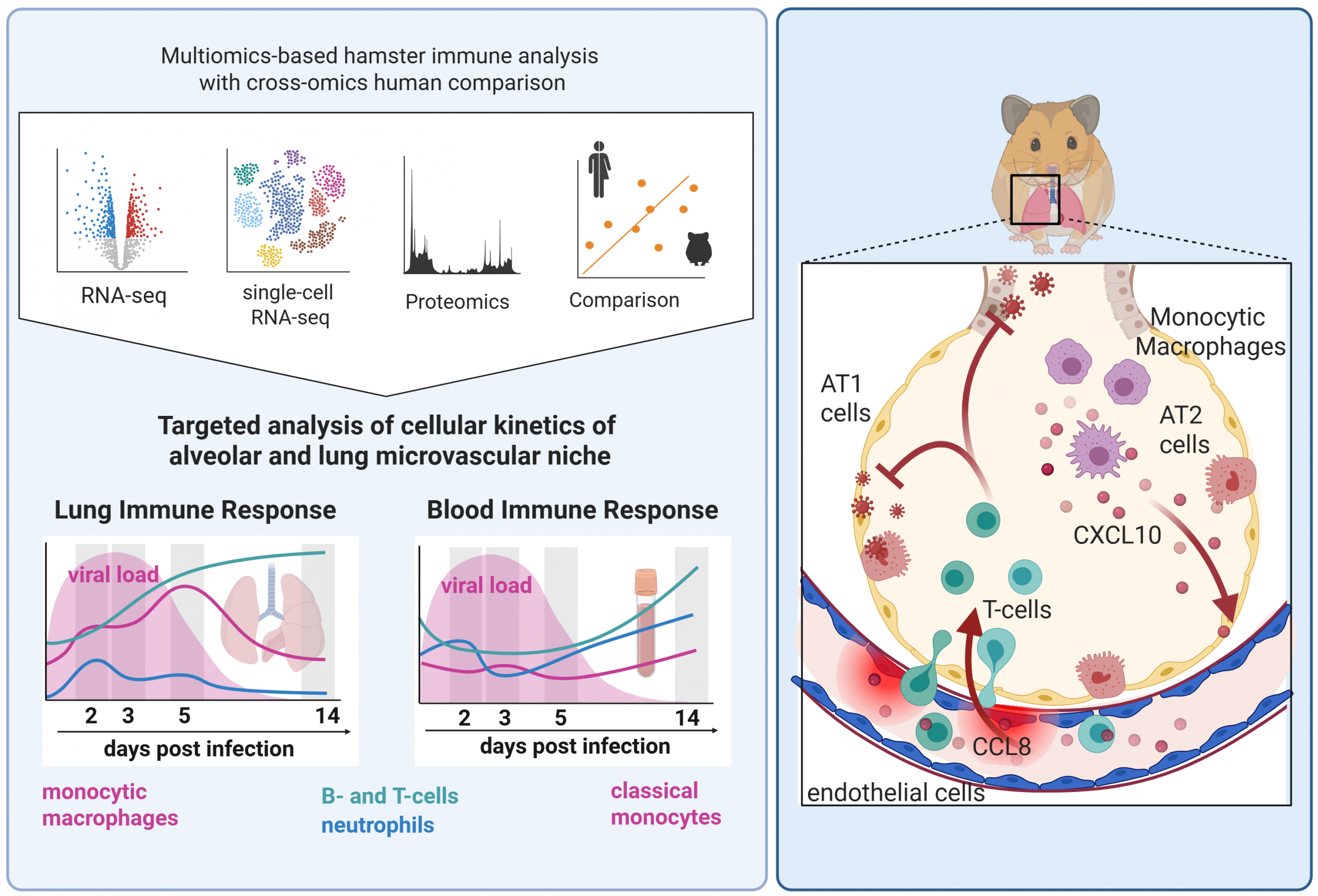
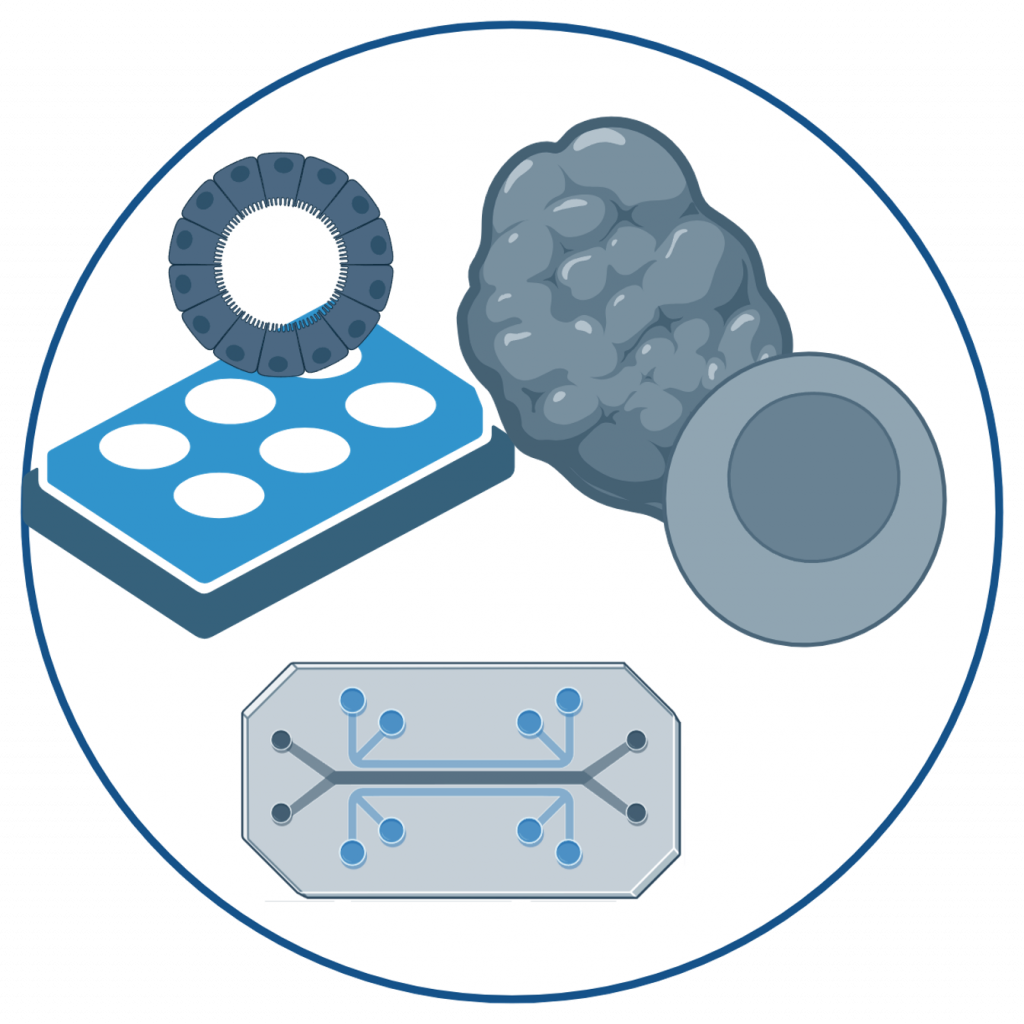
Our research in this area focuses on understanding how the innate and adaptive immune system respond to respiratory pathogens, including bacteria (Streptococcus pneumoniae, Pseudomonas aeruginosa) and viruses (SARS-CoV-2, Influenza A). We investigate the molecular mechanisms and immune cell types that contribute to both protection and pathology during infection.
To achieve this, we combine functional assays with advanced omics technologies – including spectral flow cytometry, single-cell transcriptomics, and spatial transcriptomics. Through bioinformatic analysis, we map cell-to-cell communication networks and identify immune mechanisms that are either protective or detrimental. These insights help us define correlates of protection, supporting the development of effective vaccines and experimental therapies.
Project and related links
MAPVAP: Project site at the BMBF
MAPVAP: Project site at the Institut Pasteur
CAPSyS: Systemmedizin der ambulant erworbenen Pneumonie (BMBF)
Projektbeschreibung CAPSys (Universität Leipzig)
Trimodulin description at biotest.com
Project members
Geraldine Nouailles
Peter Pennitz
Ulrike Behrendt
Chantal Weissfuss (Alumni)
Cengiz Gökeri (Alumni)
Sarah Berger (Alumni)
Romina Bischoff (Alumni)
Kerstin Linke (Alumni)
Alexander Taylor (Alumni)
Main cooperation partners
Martin Witzenrath and team
Jakob Trimpert and team
Emanuel Wyler/Markus Landthaler and team
Holger Kirsten/Markus Scholz and team
Michael Mülleder and team
Achim Gruber and team
Bastian Opitz and team
MAPVAP consortium
CAPSyS consortium
Anca Dorhoi
Stefan HE Kaufmann
Biotest AG
…many more
Project-related publications
Berger S#, Goekeri C#, Pennitz P#, Gutbier B, Michalick L, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Wienhold SM, Behrendt U, Taylor A, Dietert K, Kirsten H, Kunder S, Mueller K, Weigel M, Hain T, Volkers SM, Weis S, Gruber AD, Sander LE, Kuebler WM, CAPSyS Study Group, Witzenrath M, Nouailles G. Neutrophil-chemoattractant CXCL5 increases lung barrier permeability in acute lung injury. Mucosal Immunol. 2025 Oct 10:S1933-0219(25)00105-9. #shared first.
Nouailles G, Bischoff R, Linke K, Taylor A, Gutbier B, Pennitz P, Goekeri C, Kunder S, Voß A, Broemel T, Kershaw O, Milek M, Farztdinov V, Mülleder M, Hein CC, Visser M, Boehlaender F, Ahrens K, Beule D, Gruber AD, Koenig M, Witzenrath M. Trimodulin Supports Antibacterial Defense and Restricts Inflammation in Preclinical Pneumonia Models. Eur Respir J. 2025 2500392
Weissfuss C, Li J, Behrendt U, Hoffmann K, Bürkle M, Krishnamoorthy G, Korf IHE, Rohde C, Gaborieau B, Debarbieux L, Ricard J-D, Witzenrath M, Felten M, Nouailles G. Adjunctive phage therapy improves antibiotic treatment of Ventilator-Associated-Pneumonia with Pseudomonas aeruginosa. Nat Commun. 2025;16:4500.
Bürkle M, Korf IHE, Lippegaus A, Krautwurst S, Rohde C, Weissfuss C, Nouailles G, Tene XM, Gaborieau B, Ghigo JM, Ricard JD, Hocke AC, Papenfort K, Debarbieux L, Witzenrath M, Wienhold SM, Krishnamoorthy G. Phage-phage competition and biofilms affect interactions between two virulent bacteriophages and Pseudomonas aeruginosa. ISME J. 2025:wraf065.
Fiocca Vernengo F, Röwekamp I, Boillot L, Caesar S, Dörner PJ, Tarnowski B, Gutbier B, Nouailles G, Fatykhova D, Hellwig K, Witzenrath M, Hocke AC, Klatt AB, Opitz B. Diabetes impairs IFNγ-dependent antibacterial defense in the lungs. Mucosal Immunol. 2025;18(2):431-440.
Berger S*, Goekeri C*, Pennitz P*, Gutbier B, Michalick L, Hoffmann K, Lopez-Rodriguez E, Gluhovic V, Wienhold SM, Behrendt U, Taylor A, Dietert K, Kirsten H, Kunder S, Mueller K, Weigel M, Hain T, Volkers SM, Weis S, Gruber AD, Sander LE, Kuebler WM, CAPSyS study group, Witzenrath M, Nouailles G. Neutrophil-chemoattractant CXCL5 induces lung barrier permeability in acute lung injury. bioRxiv. 2024. *shared first
Schirm S, Nouailles G, Kirsten H, Trimpert J, Wyler E, Teixeira Alves LG, Landthaler M, Ahnert P, Suttorp N, Witzenrath M, Scholz M. A biomathematical model of SARS-CoV-2 in Syrian hamsters. Sci Rep. 2024;14(1):30541.
Friedrich VD*, Pennitz P*, Wyler E, Adler JM, Postmus D, Müller K, Teixeira Alves LG, Prigann J, Pott F, Vladimirova D, Hoefler T, Goekeri C, Landthaler M, Goffinet C, Saliba AE, Scholz M, Witzenrath M, Trimpert J, Kirsten H#, Nouailles G#. Neural network-assisted humanisation of COVID-19 hamster transcriptomic data reveals matching severity states in human disease. EBioMedicine. 2024;108:105312. *,#: equal contribution.
Peidli S*, Nouailles G*, Wyler E, Adler JM, Kunder S, Voß A, Kazmierski J, Pott F, Pennitz P, Postmus D, Teixeira Alves LG, Goffinet C, Gruber AD, Blüthgen N, Witzenrath M, Trimpert J#, Landthaler M#, Praktiknjo SD#. Single-cell-resolved interspecies comparison shows a shared inflammatory axis and a dominant neutrophil-endothelial program in severe COVID-19. Cell Rep. 2024;43(6):114328. *,#: equal contribution.
Adler JM, Martin Vidal R, Langner C, Vladimirova D, Abdelgawad A, Kunecova D, Lin X, Nouailles G, Voss A, Kunder S, Gruber AD, Wu H, Osterrieder N, Kunec D, Trimpert J. An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission. Nat Commun. 2024;15(1):995.
Nouailles G#, Adler JM#, Pennitz P, Peidli S, Alves LGT, Baumgardt M, Bushe J, Voss A, Langenhagen A, Langner C, Vidal RM, Pott F, Kazmierski J, Ebenig A, Lange MV, Mühlebach MD, Goekeri C, Simmons S, Xing N, Abdelgawad A, Herwig S, Cichon G, Niemeyer D, Drosten C, Goffinet C, Landthaler M, Blüthgen N, Wu H, Witzenrath M, Gruber AD, Praktiknjo SD, Osterrieder N, Wyler E*, Kunec D*, Trimpert J*. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat Microbiol. 2023;8(5):860-874. #shared first, *shared last
Goekeri C, Pennitz P, Groenewald W, Behrendt U, Kirsten H, Zobel CM, Berger S, Heinz GA, Mashreghi M-F, Wienhold S-M, Dietert K, Dorhoi A, Gruber AD, Scholz M, Rohde G, Suttorp N, CAPNETZ Study Group, Witzenrath M*, Nouailles G*. MicroRNA-223 Dampens Pulmonary Inflammation during Pneumococcal Pneumonia. Cells. 2023;12(6):959. *shared last
Weissfuss C, Wienhold SM, Bürkle M, Gaborieau B, Bushe J, Behrendt U, Bischoff R, Korf IHE, Wienecke S, Dannheim A, Ziehr H, Rohde C, Gruber AD, Ricard JD, Debarbieux L, Witzenrath M, Nouailles G. Repetitive Exposure to Bacteriophage Cocktails against Pseudomonas aeruginosa or Escherichia coli Provokes Marginal Humoral Immunity in Naïve Mice. Viruses. 2023;15(2):387
Pennitz P, Goekeri C, Trimpert J, Wyler E, Ebenig A, Weissfuss C, Mühlebach MD, Witzenrath M, Nouailles G. Protocol to dissociate healthy and infected murine- and hamster-derived lung tissue for single-cell transcriptome analysis. STAR Protoc. 2022;4(1):101957
Pennitz P, Kirsten H, Friedrich VD, Wyler E, Goekeri C, Obermayer B, Heinz GA, Mashreghi MF, Büttner M, Trimpert J, Landthaler M, Suttorp N, Hocke AC, Hippenstiel S, Tönnies M, Scholz M, Kuebler WM, Witzenrath M, Hoenzke K, Nouailles G. A pulmonologist’s guide to perform and analyse cross-species single lung cell transcriptomics. Eur Respir Rev. 2022;31(165):220056.
Wyler E, Adler JM, Eschke K, Teixeira Alves G, Peidli S, Pott F, Kazmierski J, Michalick L, Kershaw O, Bushe J, Andreotti S, Pennitz P, Abdelgawad A, Postmus D, Goffinet C, Kreye J, Reincke SM, Prüss H, Blüthgen N, Gruber AD, Kuebler WM, Witzenrath M, Landthaler M, Nouailles G, Trimpert J. Key benefits of dexamethasone and antibody treatment in COVID-19 hamster models revealed by single-cell transcriptomics. Mol Ther. 2022;30(5):1952-1965.
Ebenig A, Muraleedharan S, Kazmierski J, Todt D, Auste A, Anzaghe M, Gömer A, Postmus D, Gogesch P, Niles M, Plesker R, Miskey C, Gellhorn Serra M, Breithaupt A, Hörner C, Kruip C, Ehmann R, Ivics Z, Waibler Z, Pfaender S, Wyler E, Landthaler M, Kupke A, Nouailles G, Goffinet C, Brown RJP, Mühlebach MD. Vaccine-associated enhanced respiratory pathology in COVID-19 hamsters after TH2-biased immunization. Cell Rep. 2022;40(7):111214.
Andreotti S, Altmüller J, Quedenau C, Borodina T, Nouailles G, Teixeira Alves LG, Landthaler M, Bieniara M, Trimpert J, Wyler E. De Novo-Whole Genome Assembly of the Roborovski Dwarf Hamster (Phodopus roborovskii) Genome: An Animal Model for Severe/Critical COVID-19. Genome Biol Evol. 2022;14(7):evac100.
Nouailles G, Wyler E, Pennitz P, Postmus D, Vladimirova D, Kazmierski J, Pott F, Dietert K, Muelleder M, Farztdinov V, Obermayer B, Wienhold SM, Andreotti S, Hoefler T, Sawitzki B, Drosten C, Sander LE, Suttorp N, Ralser M, Beule D, Gruber AD, Goffinet C, Landthaler M, Trimpert J, Witzenrath M. Temporal omics analysis in Syrian hamsters unravel cellular effector responses to moderate COVID-19. Nat Commun. 2021;12(1):4869.
Jahn K, Handtke S, Palankar R, Weißmüller S, Nouailles G, Kohler TP, Wesche J, Rohde M, Heinz C, Aschenbrenner AF, Wolff M, Schüttrumpf J, Witzenrath M, Hammerschmidt S, Greinacher A. Pneumolysin induces platelet destruction, not platelet activation, which can be prevented by immunoglobulin preparations in vitro. Blood Adv. 2020;4(24):6315-6326.
Schirm S, Ahnert P, Berger S, Nouailles G, Wienhold SM, Müller-Redetzky H, Suttorp N, Loeffler M, Witzenrath M, Scholz M. A biomathematical model of immune response and barrier function in mice with pneumococcal lung infection. PLoS One. 2020;15(12):e0243147.
Arrey F, Löwe D, Kuhlmann S, Kaiser P, Moura-Alves P, Krishnamoorthy G, Lozza L, Maertzdorf J, Skrahina T, Skrahina A, Gengenbacher M, Nouailles G*, Kaufmann SHE*. Humanized Mouse Model Mimicking Pathology of Human Tuberculosis for in vivo Evaluation of Drug Regimens. Front Immunol. 2019;10:89. *shared last
Berger S, Goekeri C, Gupta SK, Vera J, Dietert K, Behrendt U, Lienau J, Wienhold SM, Gruber AD, Suttorp N, Witzenrath M, Nouailles G. Delay in antibiotic therapy results in fatal disease outcome in murine pneumococcal pneumonia. Crit Care. 2018;22(1):287.
Schirm S, Ahnert P, Wienhold S, Mueller-Redetzky H, Nouailles-Kursar G, Loeffler M, Witzenrath M, Scholz M. A Biomathematical Model of Pneumococcal Lung Infection and Antibiotic Treatment in Mice. PLoS One. 2016;11(5):e0156047.
Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J 3rd, Faé KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D, Mollenkopf HJ, Vogelzang A, Meyer-Schwesinger C, Mittrücker HW, McEwen G, Kaufmann SH. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124(3):1268-82.
Dorhoi A, Iannaccone M, Maertzdorf J, Nouailles G, Weiner J 3rd, Kaufmann SH. Reverse translation in tuberculosis: neutrophils provide clues for understanding development of active disease. Front Immunol. 2014;5:36.
Dorhoi A, Iannaccone M, Farinacci M, Faé KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf HJ, Oberbeck-Müller D, Jörg S, Heinemann E, Hahnke K, Löwe D, Del Nonno F, Goletti D, Capparelli R, Kaufmann SH. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013;123(11):4836-48.
Editorials and press
Phages and antibiotics work better together against pneumonia (Paper Spotlight – Charité, 16.5.2025)
Phage Therapy Boosts Antibiotics Against Ventilator Pneumonia (Sciencemag, 15.05.2025)
How AI is helping to bridge the research gap between animals and humans (idw, 25.09.2024)
A new approach to a Covid-19 nasal vaccine shows early promise (CNN, 03.04.2023).
Nasal vaccine to prevent COVID-19 passes first tests (upi.com, 03.04.2023).
Nasenimpfstoff gegen Corona erfolgreich getestet (aerzteblatt.de, 04.04.2023).
Kommt die Nasenspray-Impfung gegen Corona? (zdfheute, 04.04.2023).
Impfung per Nasenspray könnte Pandemie beenden (Berliner Zeitung, 18.05.2022)
Corona-Impfung per Nasenspray: Schnief und Schluss (Zeit-Online, 19.05.2022)
Lebendimpfstoff aus Berlin erzeugt Schleimhautimmunität (Spektrum.de, 18.05.2022)
Nasale Corona-Impfstoffe: Hoffnung auf sterile Immunität (Deutschlandfunk, 18.05.2022)
Neues Nasenspray aus Berlin soll besser als RNA-Impfung sein (mdr.de, 19.05.2022)
Spray könnte besser wirken als Spritze (blick.ch, 20.05.2022)
Corona-Impfung: Forscher sehen Erfolge im Tierversuch (sueddeutsche.de, 18.05.2022)
This research area focusses on the long-term consequences of respiratory infections. We investigate how pneumonia can aggravate cardiovascular conditions such as atherosclerosis, as well as how infections may contribute to post-infectious neuropsychiatric disorders.
By combining clinical observations with experimental models, we aim to uncover the mechanisms that link acute infections to long-term health consequences. These insights will help identify pathways and potential targets for prevention and therapy.
Project and related links
RTG NeurInfect is funded by the DFG
Project members
Geraldine Nouailles
Peter Pennitz
Julia Schumann (starting March)
Jo Bagli (Alumni)
Richard Steinhorst (Alumni)
Main cooperation partners
Sebastian Weis and team
Nils Opel and team
Arash Haghikia and team
Wolfgang Kübler, Szandor Simmons
Martin Witzenrath, Markus Brack and team
Markus Scholz, Holger Kirsten and team
Project-related publications
Ramezani Rad P, Nageswaran V, Peters L, Reinshagen L, Roessler J, Simmons S, Asmus E, Wittig C, Brack MC, Nouailles G, van der Vorst EPC, Maas SL, Sonnenschein K, Verhaar BJH, Szulcek R, Witzenrath M, Landmesser U, Kuebler WM, Haghikia A. Pneumonia Induced Rise in Glucagon Promotes Endothelial Damage and Thrombogenicity. Circ Res. 2024;135(11):1116-1118.
Schirm S, Haghikia A, Brack M, Ahnert P, Nouailles G, Suttorp N, Loeffler M, Witzenrath M, Scholz M. A biomathematical model of atherosclerosis in mice. PLoS One. 2022;17(8):e0272079.
Schirm S, Ahnert P, Berger S, Nouailles G, Wienhold SM, Müller-Redetzky H, Suttorp N, Loeffler M, Witzenrath M, Scholz M. A biomathematical model of immune response and barrier function in mice with pneumococcal lung infection. PLoS One. 2020;15(12):e0243147.
Conference Papers
e:Med Meeting 2024, 21 – 23.11.2024, Hamburg. Brack M, Steinhorst R, Pennitz P, Mueller K, Nouailles G, Teixeira Alves LG, Wyler E, Witzenrath M, Scholz M, Kirsten H. From Lung to Heart: scRNA-seq Illuminates Post-Pneumonia Cardiovascular Risks.
American Thoracic Society 2024 International Conference, 17 – 22.05.2024, San Diego, USA. Melnikov A, Solymosi PD, Pennitz P, Schupp JC, Nouailles G, Perret PL, Gallo K, Hegemann N, Nambiar Veetil N, Klepetko W, Sinn K, Erfinanda L, Simmons S, Kucherenko MM, Knosalla C, Preissner R, Kwapiszewska G, Witzenrath M, Kuebler WM. Cellular Mechanisms Underlying the Pathogenetic Role of the Primary Cilium in Pulmonary Arterial Hypertension (abstract). Am J Respir Crit Care Med 2024;209:A6790. DOI: https://doi.org/10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A6790
e:Med Meeting 2023, 10 – 11.10.2023, Berlin. Pennitz P*, Friedrich VD*, Wyler E, Adler JM, Postmus D, Andreotti S, Teixeira Alves LG, Kazmierski J, Pott F, Hoefler T, Goekeri C, Landthaler M, Goffinet C, Trimpert J, Scholz M, Witzenrath M, Kirsten H#, Nouailles G#. Interspecies analysis to dissect transcriptomic signatures of humans and hamsters in COVID-19.
e:Med Meeting 2022, 28 – 30.11.2022, Heidelberg. Pennitz P*, Kirsten H*, Friedrich VD, Wyler E, Goekeri C, Obermayer B, Heinz GA, Mashreghi MF, Büttner M, Trimpert J, Landthaler M, Suttorp N, Hocke AC, Hippenstiel S, Tönnies M, Scholz M, Kuebler WM, Witzenrath M, Hoenzke K, Nouailles G. Integrational approaches for cross-species analysis of lung pathologies at single-cell resolution.
SCOG Conference “Single Cell Omics in Clinical Applications”, 07 – 09.11.2022, Bonn. Pennitz P *, Friedrich VD *, Wyler E, Adler JM, Postmus D, Andreotti S, Teixeira-Alves LG, Kazmierski J, Pott F, Hoefler T, Goekeri C, Landthaler M, Goffinet C, Trimpert J, Scholz M, Witzenrath M, Kirsten H#, Nouailles G#. Interspecies analysis to dissect cellular transcriptomic signatures of humans and hamsters in COVID-19.
21st International Conference on Systems Biology, 08.-12.10.2022, Berlin. Pennitz P*, Kirsten H*, Friedrich VD, Wyler E, Goekeri C, Obermayer B, Heinz GA, Mashreghi MF, Büttner M, Trimpert J, Landthaler M, Suttorp N, Hocke AC, Hippenstiel S, Tönnies M, Scholz M, Kuebler WM, Witzenrath M, Hoenzke K, Nouailles G. Integrational approaches for cross-species analysis of lung pathologies at single-cell resolution.